Quality management according to ISO 9001
A quality management system (QMS), according to ISO 9001 for example, is a genuine management and control system. Therefore, the decision to introduce such a system forms part of a company's strategic decisions.
A quality management system (QMS) is deemed to prove that a company is capable of guaranteeing at any time the defined quality of its products and services. The company is thus able to focus more on its customers in order to acquire competitive advantages. The aim of a QMS is not only short-term tangible profits: a QMS acts on a medium- and long-term basis.
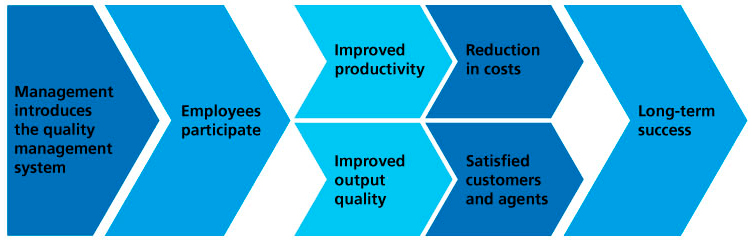
Chain reaction
The chain reaction of a QMS can be defined as follows:
What do we mean by quality?
Quality is designated by “all of an organization’s economic and non-pollutant measures aimed at meeting the expressed or implicit needs of customers and of the company”.
How much quality? The (external) quality expressed by the customer is necessary to a company’s survival. But this is not enough. The company must guarantee that quality is achieved at prices consistent with the market (internal quality).
Quality management has therefore become necessary, since it costs less to prevent errors than to correct them at the end of the production process. Costs associated with errors are minimized when the company masters its processes, which are decisive for quality, and continuously improves its performances.
ISO 9001
The ISO 9001 standard is the leading QMS certification worldwide. It describes in exemplary fashion the quality management system as a whole and serves as the basis for constituting a comprehensive QMS. You will find below the seven principles of quality management, described according to ISO 9001:
- Customer focus
- Leadership
- Engagement of personnel
- Process approach
- Improvement
- Evidence-based decision-making
- Relationship management
Main scope
Three aspects are hugely important for the introduction of ISO 9001:
- Market strategy
In terms of market strategy, ISO certification is used by a company placed among the competition to prove the quality of its products and services. For manufacturers, suppliers and large international companies, certification is “virtually mandatory” to access mandates of a certain scale.
- A guaranteed future
The introduction of a QMS helps a company continue to develop its own potential. This is an excellent way for a company to organize and direct its future with greater peace of mind, despite change and modified framework conditions and requirements.
- Legal significance
In legal terms, the standard ISO 13485 (medical products) and the ISO 9000 series (any other product) are accepted as the single basis for certification by all national standardization and certification companies in Europe and worldwide.
Therefore, these standards present a very broad legal base. This is hugely important to companies operating on an international scale, particularly for product liability It is important to remember however, that standards are not, in themselves, legal in nature.
Fundamental rules of a QMS
A management system is an instrument making it possible to apply a company’s objectives in a targeted way. This means planning company procedures, executing these according to the planning, controlling success and correcting these if the desired result is not achieved.
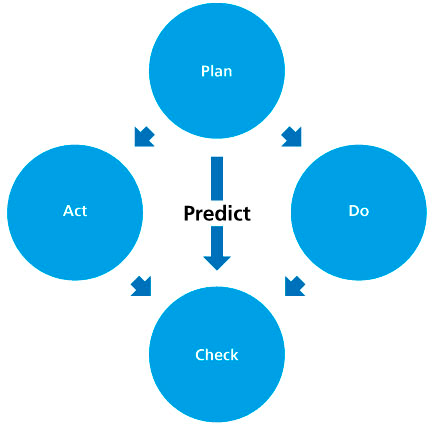
Whilst the quality management system is represented by a diagram, it represents a PDCA cycle (plan, do, check, act). Moreover, it is necessary to begin with an analysis of the current state of the company – situational analysis – hence the term A-PDCA (A for analysis).
The cycle works in this way:
- During the preliminary phase, the plan is drawn up, which describes what, how and who is responsible for execution of the task. At the same time, a prediction is given as an objective, the expected result of the task.
- During the implementation phase, execution takes place according to the plan. A sub-cycle of rules can be introduced, in the case of large tasks/projects. The results are defined as effective objectives.
- At the time of control, the prediction and the effective objective are compared.
Adaptation measures are examined on the basis of the evaluation of the comparison:
- Does execution need to be modified?
- Does the plan need to be modified?
- Are new predictions necessary?
- etc.